-40%
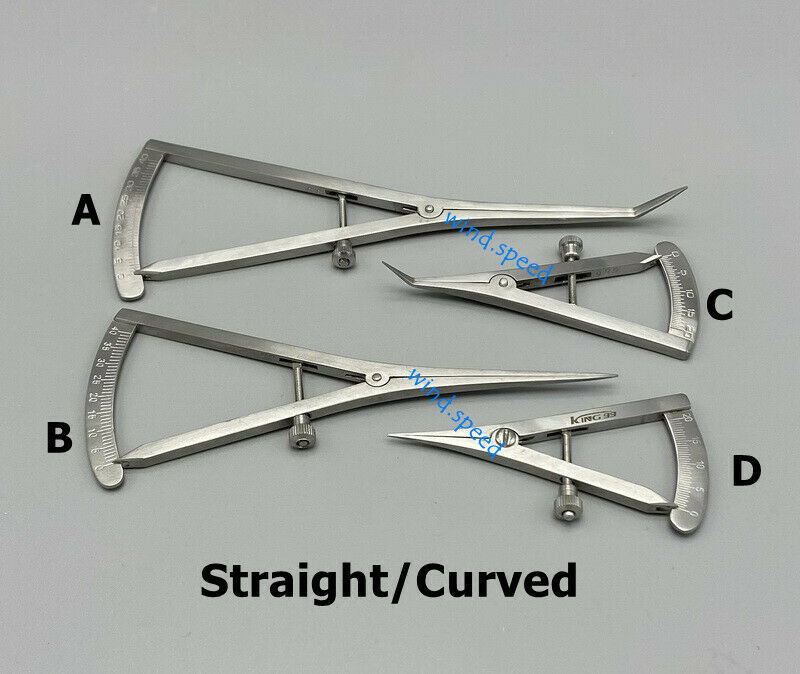
Dental Surgical Instrument Implantology Caliper Implant Gauge 12.5 CM
$ 24.28
- Description
- Size Guide
Description
Dental Surgical Instrument Implantology Caliper Implant Gauge for surgical procedures12.5 CM
.
Material: Stainless Steel
Made in Germany
Instructions for Sterilization and Instrument Care
General requirements for the preparation of medical devices
Please observe the valid legal regulations of your country as well as the hygiene guidelines for
practices and clinics.
■
In particular, please comply with the guidelines regarding the effective inactivation of prions.
The ever-present risk of contamination and infection during treatment must be excluded or
reduced by specific preventive measures. These include:
• Assessment of the risks and dangers in connection with medical practice and definition of
appropriate precautions.
• Schematization and systematization of work routines with the predominant aim of
avoiding contamination and injuries.
• Careful medical history with special regard to patient-induced risks of infection.
■
Not only do used medical devices have to be considered contaminated, but those which are
openly displayed must therefore also be prepared hygienically without exception.
During transport it has to be assured that neither staff nor third parties are at risk. For their
own safety, staff members must wear appropriate protective clothing and protective gloves.
■
Medical devices should not be stored in physiological saline solution as prolonged exposure
may lead to corrosion. The parts must be moistened completely and bubble-free in a bath.
Demineralized water is required under all circumstances for final rinsing after disinfection
to avoid water stains and crystal seeding which may interfere with the subsequent process of
sterilization.
■
As your responsibility includes the sterility of the medical devices used, please bear in mind
that only validated procedures must be used for cleaning, disinfection and sterilization. All
equipment needs to be serviced at regular intervals, and all parameters must be observed
in every cycle. Please observe the shelf-life of the sterile packaging of products (see
manufacturer‘s instructions). Preparation ends with the release for use.
■
Every sterile packaging should display a sterilization indicator and be marked with the date
of sterilization.
■
For medical devices where is not named as manufacturer, please check the specific guidelines
regarding preparation and re-usability in the manufacturer‘s instructions for use.
■
All removable parts must be disassembled for cleaning and disinfection and assembled prior
to sterilization. In case of removable parts, please only clean and disinfect the parts of a single
instrument in a batch.
■
If using an ultrasonic bath, the drills must be placed in a drill organizer.
3
Distinguish between manual and mechanical procedures for preparation, whereby the
mechanical procedure is always to be preferred. The following gives a guide line for mechanical
and manual preparation as well as for sterilization.
Mechanical preparation
Manual pre-cleaning is necessary to provide efficient mechanical cleaning, so that the
mechanical reprocessing of medical devices begins with a manual cleaning step.
1. Manual pre-cleaning:
Coarse contamination such as blood, tissue and bone residue must be removed immediately
after use. To this purpose the instruments are to be removed from the instrument tray and
rinsed under cold running water while removing coarse contamination with a fine, soft nylon
brush. Then the instruments which are to be cleaned mechanically are placed in the cleaning
basket of cleaning and disinfection.
Washer-disinfectors units
1.1 a. Cleaning of medical devices with through-holes, for example, irrigation cannulas or
cooling holes:
Due to the small diameters of the through-holes, medical devices with cooling holes or drills
with internal irrigation make high demands on reprocessing. To ensure safe preparation of such
delicate instruments, these need to be pre-cleaned manually. Hereby it is essential to observe
the following points:
■
Immediately after use, the capillary is to be cleaned from coarse contamination using a Miller
needle and checked for obstructions.
■
Holes are to be rinsed several times with distilled water using a disposable syringe (10 ml).
■
Medical devices may then be cleaned mechanically.
■
Holes are to be rinsed several times with distilled water after mechanical cleaning using a
disposable syringe (10 ml).
1.1 b. Depth Stops for Twist Drills
Disassembly
• Place the tip of the drill on a soft pad (e.g. surgical cloth)
• Now please remove the depth stop manually over the tip of the drill
1.2 Ultrasonic cleaning (optional):
• If the medical devices are heavily soiled or if coarse contamination proves difficult to
remove manually (as described above), pre-cleaning in an ultrasonic bath-is recommended.
• Care should be taken to ensure that the cleaning agent is compatible with the products and
that the exposure times and concentrations recommended by the manufacturer are adhered
to.
• Please also ensure that the liquid levels recommended by the manufacturer of the
ultrasonic bath are adhered to.
4
2. Mechanical cleaning:
Suitable cleaning and disinfecting appliances Washer-disinfectors units are to be used which
are to be validated by the user as part of the established cleaning processes. An example for a
suitable unit is the following one together with appropriate accessories.
Notes on loading
Instrument set is inserted into the cleaning/disinfecting appliance.
■
The instruments to be cleaned, or their disassembled components, are placed in the
destination baskets.
■
The cover of the cleaning basket should be closed.
The instructions of the Washer-disinfectors unit manufacturer are to be followed. The cleaning
and disinfecting agents shall comply. Cleaning and neutralization agents are to be dosed and
used according to the manufacturer‘s guidelines. The water quality recommended for cleaning
(especially for the final rinse phase) is fully demineralized water or water which corresponds
to this level of purity. The recommended cleaning program is the program with thermal
disinfection which operates at the optimum temperature of 45-55°C for the removal of blood, or
any other adequate and validated program.
The program is divided into the following phases:
• Pre-rinse with cold water 4 min.
• Cleaning with alkaline detergent at max.55°C 10 min.
• Neutralization 6 min.
• Intermediate rinse 3 min.
• Disinfection 5 min.
• Drying 30 min.
3. Thermal disinfection:
Thermal disinfection is part of the program and takes into account the value. The latter is a
measure for the reduction of microorganisms in steam disinfection processes.
4. Drying prior to sterilization:
The instruments are dried automatically during the drying cycle in the cleaning and
disinfecting appliance. Clean compressed air can be used to dry drill holes/capillaries. Then the
cleaned and disinfected parts are to be checked again for damage and any corrosion.
Manual preparation
1. Pretreatment (to avoid cross contamination)
Attention: dried contamination may severely impede the reprocessing of medical devices.
One should therefore start pretreatment as early as possible. To prevent drying and for reasons
of personal protection (bactericidal, fungicidal, sporicidal and antiviral effects), medical devices
5
are to be placed in disinfectant solution immediately after use** (shake thoroughly at least three
times). Then, coarse contamination such as blood, tissue and bone residue must be removed.
To this purpose the instruments are to be removed from the instrument tray and all visible
remains of surgery and adhering contamination are to be rinsed off under cold running water
or in a disinfectant solution with a fine, soft brush (nylon) or a clean, soft cloth which is only
used for this purpose. Never use metal brushes or steel wool. Cleaning/disinfection should then
take place within the next 2 hours.
Attention:
At temperatures exceeding 40°C there is a risk of protein coagulation. The optimum operating
temperature for a disinfectant bath is room temperature. This initial disinfection cannot replace
the disinfection step which is carried out after cleaning!
Оptional:
1. Ultrasonic cleaning :
If the medical devices are heavily soiled or if coarse contamination proves difficult to remove
manually as described above), pre-cleaning in an ultrasonic bath-is recommended. Care
should be taken to ensure that the cleaning agent is compatible with the products and that the
exposure times and concentrations recommended by the manufacturer are adhered to. Please
also ensure that the liquid levels recommended by the manufacturer of the ultrasonic bath are
adhered to.
2. Cleaning
• Prior to cleaning the products are to be rinsed with running cold water.
• All removable parts are to be disassembled.
• The water quality recommended for cleaning is distilled water or fully demineralized water.
• Use a suitable cleaning agent such as an alkaline (ingredients for cleaning agents according
to EC Detergents Regulation648/2004: enzymatic, NTA, anionic surfactants).
Attention:
• Cleaning should be carried out at a maximum temperature of 40°C.
• The optimum operating temperature is room temperature.
• The disassembled medical devices are to be placed in a freshly prepared cleaning bath
in accordance with the manufacturer‘s guidelines and then cleaned with an instrument
cleaning brush made of plastic/nylon.
• Medical devices with through-holes, for example, irrigation cannulas or cooling holes
are to be cleaned with a Miller needle and rinsed several times with distilled water using
a disposable syringe (min. 10 ml) at the beginning and end of the exposure time. Ensure
there are no obstructions in the through-holes. After cleaning, the products are to be rinsed
several times with distilled or fully demineralized water.
• After cleaning, the products are to be examined for damage or any signs of corrosion.
• Damaged products are to be replaced.
6
3. Disinfection
For the disinfection of medical devices these are to be placed in a fresh disinfection bath**
(shake thoroughly at least three times).
Attention:
The products must be fully immersed/ wetted by the disinfectant solution. For example, a
suitable disinfectant with bactericidal, fungicidal and antiviral effects (HIV, HBV and HCV).
4. Rinsing and Drying
Following disinfection, the components are to be rinsed thoroughly three times with distilled
water. Drying of the instrument and the drill holes is performed using clean compressed air.
After rinsing and drying, the products are again to be examined for damage or any signs of
corrosion. Damaged products are to be replaced.
Sterilization
• Prior to sterilization, the disassembled medical devices are to be re-assembled.
• The separately cleaned and disinfected medical devices are to be sorted into the provided
sterilizable tray, but can also be sterilized individually.
• The filled trays and/or the individual medical devices are then packed in disposable
sterilization packs (single or double packs) and/or a sterilization container suitable for
steam sterilization.
• Packaging suitable for steam sterilization must comply with the requirements according
to DIN EN ISO 11607 / ANSI/AAMI ST79 / AAMI TIR12:2010, for example, disposable
sterilization packs (single or double packs) temperature resistant up to at least 135°C
(279°F) and sufficient steam permeability, which provide sufficient protection against
mechanical damage, or sterilization containers which need to be maintained according to
the manufacturer‘s instructions.
1. Sterilization
Sterilization is performed in the autoclave 7-10 minutes at 132°C to134°C or for 20 minutes
at 121°C (given are the minimum dwell times, the operating times are longer and canvary
depending on the appliance).
2. Storage
Store the sterilized components at room temperature in a dry and dust-free place.
** Observe information provided by the manufacturer of the instrument disinfection/cleaning
agent with regard to concentration/exposure time and, if given, temperature.
Only disinfectants not containing chlorine, ammonia and aldehydes and with proven efficacy
against HBV, HCV and HIV may be used, and which comply with the respective current
national regulations on disinfectants (i.e. FDA approval, DDGHM (2002)/VAH (2011)-listed, CE
label, etc.).
Disinfection should be performed using aldehyde-free agents due to the protein-binding
properties of aldehyde-containing disinfectants.
7
[1.] DIN EN ISO 17664 Sterilization of medical devices– Information to be provided by the
manufacturer for the preparation of re-sterilizable medical devices (ISO17664:2004); German
version EN ISO 17664:2004
[2.] RKI Guideline 2001: Hygiene requirements for the preparation of medical devices
(Bundesgesundheitsblatt44: 1115-1126) (German Federal Health Gazette)
[3.] Guidelines of the DGKH (German Association for Hospital Hygiene), DGSV (German
Association for Sterile Services) and AKI (Working party instrument preparation) for the
validation and routine monitoring of mechanical cleaning and thermal disinfection processes
for medical devices and the principles of equipment selection, 3rdedition 2008.
[4.] Draft Guidance for Industry and FDA Staff/Processing/Reprocessing Medical Devices in
Health Care Settings: Validation Methods and Labeling 2nd May 2011.
[5.] ANSI/AAMI TIR12 September 2010 Designing, testing, and labeling reusable medical
devices for reprocessing in health care facilities: A guide for medical device manufacturers.
[6.] DIN EN ISO 15883-1 (2006-09) Cleaning and disinfection appliances - Part 1: General
requirements,
Definitions and test methods (15883-1:2006); German version EN ISO 15883-1:2006
Trays/kits have to be conditioned after each use. Please disassemble them according to the
following instructions:
■
Please remove all products from the tray/kit for conditioning (cleaning/disinfection). Now
disassemble the tray/kit completely into its individual components.
■
Remove the base plate and the accessory box, if present, from the tray or the kit.
■
Remove the aluminum insert for separate cleaning.
■
Reassemble the cleaned and disinfected tray/kit for maintenance and assembly. Do not use
instrument oil or damaged trays.
■
Place the separately cleaned and disinfected instruments back into the tray (preparation for
sterilization).
■
Please pack the trays in disposable sterilization packs (single or double packs) and/or
sterilization containers that conform to the requirements.
■
Sterilization is carried out as described previously.
IMPORTANT!
• All medical devices which are supplied non-sterile must be sterilized before being used on
patients for the first time.
• All re-usable medical devices must be prepared and sterilized after each use in accordance
with the described procedures. If a different procedure is used, this must be validated by
the respective practice or hospital.
8
• Not all medical devices can be re-used repeatedly. Products which are not suitable for
steam sterilization due to their material composition must be subjected to disinfection
prior to use. Information on whether a product is re-usable, needs to be disassembled for
preparation, or can be sterilized in an autoclave.
Shipping Information:
The average shipping times for our orders are as follows (standard shipping):
Worldwide: 7-21 Business Days
If for some reason your order has not arrived within 40 Days, please get in contact with us so we could help you.
(After the shipping started, the order will be monitored and the track of the item will be posted on eBay
Payment:
Payment can only be made by PayPal via eBay. To ensure your purchase is protected, it will only be delivered to your registered PayPal address (Due to safety reason we do not allow change address through email). Payment for Buy It Now purchases must be made immediately. Once your payment has been received we will send you a confirmation email.
If your item needs to be shipped to an alternative address, please update the address on PayPal before placing your order. We will only ship to your registered PayPal address.
Return Policy:
We want you to be completely happy! If for any reason you are not happy with your purchase, we will gladly accept returns. You can send back a goods within 60 Days. Return shipping will be paid by Buyer.
Returned items should be in unused, not damaged or worn conditions. Returned items need to be in original packaging.
SERVICE/FEEDBACK
Questions:
Were here for you!
We are proud to offer quality service and we want your transaction to go perfectly. Take advantage of our customer service representatives who are here to help you, contact us on eBay. Thank you for your interest and we hope to do business with you! We would be thankful if You could leave good response about us.
Thank you for your interest and we hope to do business with you!